Quantum dots are very small, semiconductive particles measuring between approximately two and fifty nanometers in diameter. Electrons of a quantum dot are excited by an external light source followed by a release of energy as the particle returns to the ground state. For quantum dots, this release of energy results in the emission of fluorescent light of varying color depending on the size and composition of the particle. Due to these optical and electrical properties, quantum dots are especially useful for medical imaging purposes as well as in commercial televisions, cellular devices, and other common electronics. The setback is that most quantum dots are composed of very toxic metals such as cadmium.
This is not a problem if the quantum dots are confined. Although, once these products are discarded, the quantum dots have the potential to damage the living systems in the surrounding area by leaking into the soil and nearby bodies of water. It is certain that the quantum dots destroy the living systems due to their chemical make-up, but what is less understood is how this process takes place.
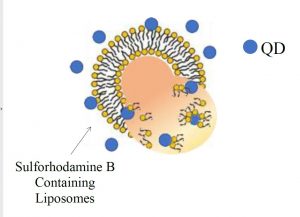
In the laboratory, liposomes are used as a model of the bacterial membrane in order that the interaction between quantum dots and living systems may be more fully understood. Using single molecule microscopy, one is able to observe a single liposome as it interacts with quantum dots in real time. Prior to imaging, the liposome is filled with a dye such as sulforhodamine B so that it is evident when the liposome has broken open since this will result in a release of the dye. More fully understanding the interaction between the two components will aid in research to prevent the continuation of the destruction of living systems on account of quantum dots.