The goal of this project is to develop a simple model for self-assembled photosynthetic light-harvesting antenna. The development of artificial photosynthetic systems has been widely explored as a solution to the consumption of fossil fuels and its corresponding problems, such as lack of sustainability, and extensive greenhouse gas emissions. Ideal artificial photosynthetic systems include a light-harvesting antenna with strong electronic coupling and efficient energy transfer for an efficient collection of solar energy. With strong electronic coupling being a characteristic of well-defined assemblies of porphyrins, this project determines the molecular orientation, electronic overlap, and absorption and fluorescence properties of self-assembled artificial chlorin dimers.
 Following the Lindsey method, artificial 13-bromo-18,18-dimethyl-10-tolyl chlorin is prepared, then subjected to Suzuki coupling reactions, to prepare a series of novel monomers. Monomers included methyl imidazole and pyridyl substituted zinc chlorin derivatives. Following synthesis, self-assembly process should lead to the formation of coordination bonds incorporating the heterocyclic nitrogen lone pair and Zn2+ of the chlorin core, favoring structurally defined coordination dimers. As a final component to the project, excitonic coupling energy and relative fluorescence intensity of the chlorin dimers can be characterized using UV-vis absorption and fluorescence measurements.
Following the Lindsey method, artificial 13-bromo-18,18-dimethyl-10-tolyl chlorin is prepared, then subjected to Suzuki coupling reactions, to prepare a series of novel monomers. Monomers included methyl imidazole and pyridyl substituted zinc chlorin derivatives. Following synthesis, self-assembly process should lead to the formation of coordination bonds incorporating the heterocyclic nitrogen lone pair and Zn2+ of the chlorin core, favoring structurally defined coordination dimers. As a final component to the project, excitonic coupling energy and relative fluorescence intensity of the chlorin dimers can be characterized using UV-vis absorption and fluorescence measurements.
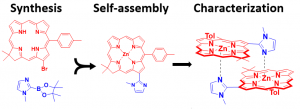
Project outline on the synthesis, self-assembly, and characterization of methyl imidazole substituted zinc chlorin dimers.